How Do Water Softeners Work?
A Water Softener will:
- End the hassle of house-hold cleaning and spots on dishware
- Save you money on wasted detergents and soap products
- Improve the quality of your clothes, skin and hair
It will also improve and protect your home’s plumbing and fixtures from scale and blockage.
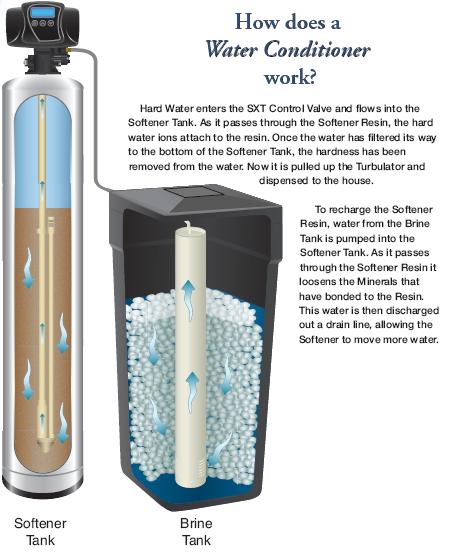
How it Works:
A Water Softener functions on ion exchange. Hard water minerals like magnesium and calcium are cations, or have a positive charge. The Water Softener has an anionic- negatively charged- resin bed. As the positively charged minerals pass through the negatively charged resin, the hard minerals are attracted to the resin beads. This ion exchange removes the hard minerals from your water, leaving soft water that is ready for use. Over time, the resin will attract all the cations it can hold and will not be able to attract more until it has been cleaned of mineral. This requires the softener to regenerate, or clean the resin.
To recharge and clean the resin, salt brine is used. The common misconception is that salt softens the water. Salt is only used to make a brine that is injected into the Water Softener, recharging the resin in a 1 to 1 exchange for the hard minerals and then flushed out. Sodium attracts the hard mineral removing them from the resin beads. This is then washed away through automatic back-washing. Regeneration timing and the amount of sodium needed depend on the use of water in your home and the hardness level of your water. Salt is the inexpensive option for regeneration. However, other products are available, such as Potassium Chloride.